Introduction to Alkali Metals
What Are Alkali Metals?
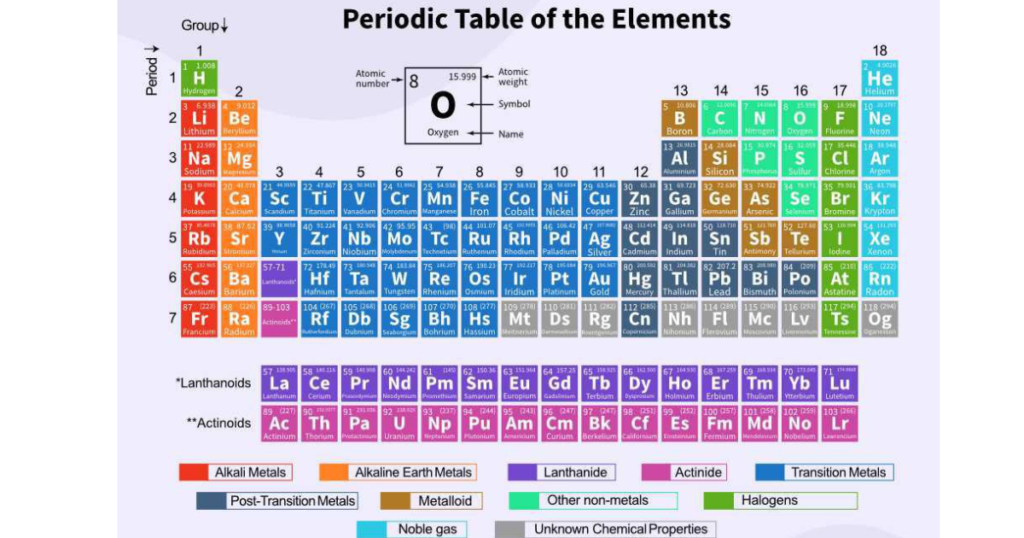
Alkali metals are a group of highly reactive elements found in Group 1 of the periodic table. This unique set includes lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). These elements share similar chemical and physical properties, primarily because they each have a single electron in their outermost energy shell. This electron configuration makes them incredibly reactive, especially with water and air, and gives them their distinct place in chemistry.
Where Are Alkali Metals Found?
In nature, alkali metals are not found in their pure elemental state due to their high reactivity. Instead, they are present in the form of various compounds. For instance, sodium exists abundantly in ocean water as part of sodium chloride (table salt), while lithium can be extracted from brines and certain minerals like spodumene.
The Periodic Table and Alkali Metals
Position of Alkali Metals in the Periodic Table
Alkali metals are positioned in the first column (Group 1) of the periodic table. This group is the starting point for the s-block elements, and it lays the foundation for understanding chemical reactivity.
Group 1 Elements Overview
All Group 1 elements are metals, characterized by their ability to lose one valence electron easily. This property contributes to their exceptional reactivity, making them essential in both scientific studies and industrial applications.
Properties of Alkali Metals
Physical Properties
Softness and Density
One striking feature of alkali metals is their softness. You can literally cut them with a knife! They also have low densities compared to other metals, with lithium being the least dense solid element.
Lustrous Appearance
When freshly cut, alkali metals display a shiny, silvery luster. However, this appearance doesn’t last long as they quickly tarnish upon exposure to air, forming oxides.
Chemical Properties
Reactivity with Water
Alkali metals react vigorously with water to produce hydrogen gas and a hydroxide compound. For instance, when sodium interacts with water, it forms sodium hydroxide and releases hydrogen gas, often with enough heat to ignite the gas.
Tendency to Form Ions
These elements lose their single valence electron with ease, forming positively charged ions (cations). This is why they are so reactive, particularly with nonmetals like halogens, to form ionic compounds.
READ MORE:Top 10 Cheapest Metals In the World
The Members of the Alkali Metals Family

Lithium (Li)
Lithium, the lightest alkali metal, is widely used in rechargeable batteries for electronics like smartphones and laptops. It’s also an important component in mood-stabilizing medications, making it invaluable in the fields of technology and medicine.
Sodium (Na)
Sodium is a cornerstone of daily life. Its most well-known compound, sodium chloride, is the table salt that flavors our food. Sodium also plays a significant role in chemical manufacturing, including the production of detergents and glass.
Potassium (K)
Potassium is crucial for life. It helps regulate fluid balance, nerve signals, and muscle contractions in our bodies. Agriculturally, potassium-based fertilizers are vital for enhancing plant growth and crop yield.
Rubidium (Rb)
Although less common, rubidium is used in specialized applications like atomic clocks and in the production of certain types of glass. Its unique properties also make it useful in research fields.
Cesium (Cs)
Cesium is a highly reactive metal with specialized uses in timekeeping. Cesium-based atomic clocks are the most accurate clocks ever created, often used for GPS systems and scientific experiments.
Francium (Fr)
Francium is the rarest and most unstable of all alkali metals. With a half-life of just 22 minutes, it exists only in trace amounts in nature and is primarily studied in research labs.
Applications of Alkali Metals

Industrial Uses
Alkali metals play a pivotal role in industry. Sodium and potassium compounds are extensively used in glass manufacturing, soap production, and the synthesis of various chemicals. Lithium, on the other hand, powers our modern world by being the backbone of rechargeable batteries.
Role in Medicine
Lithium salts are crucial in treating bipolar disorder, while potassium is indispensable for maintaining a healthy electrolyte balance. Additionally, sodium is a key component in intravenous fluids and medications.
Everyday Applications
From the salt we sprinkle on our meals to the batteries that power our devices, alkali metals are everywhere. Sodium vapor lamps illuminate streets at night, and potassium is a key ingredient in fertilizers that sustain agriculture.
Hazards and Precautions
Dangers of Reactivity
Alkali metals are not substances you can handle casually. Their high reactivity, especially with water and air, makes them dangerous. Even a small piece of sodium dropped into water can cause an explosive reaction.
Safe Storage Practices
To prevent accidental reactions, alkali metals are stored under oil or in inert gases like argon. This keeps them isolated from air and moisture, ensuring safety.
Fun Facts About Alkali Metals
- Cesium clocks are so accurate that they only lose one second in millions of years.
- Lithium is the only alkali metal that reacts with nitrogen under normal conditions.
- Francium is so rare that scientists estimate there are less than 30 grams of it on Earth at any given moment.
- Potassium’s iconic purple flame color is often used in fireworks displays.
- Rubidium can ignite spontaneously in air due to its extreme reactivity.
Conclusion
Alkali metals are truly the reactive marvels of the periodic table. From lithium’s role in powering our gadgets to potassium’s contribution to our health, these elements are indispensable in modern life. However, their reactivity also demands careful handling and storage. As we continue to explore their properties, applications, and potential, alkali metals remind us of the fascinating balance between utility and danger in the world of chemistry.
FAQs
1.What makes alkali metals so reactive?
Alkali metals are reactive because they have only one electron in their outermost shell, which they readily lose to form positive ions, resulting in highly exothermic reactions.
2.Why are alkali metals stored in oil?
Alkali metals are stored in oil to prevent them from reacting with air or moisture, which could lead to dangerous reactions.
3.What are some common uses of alkali metals?
Common uses include lithium in batteries, sodium in salt and detergents, and potassium in fertilizers and biological systems.
4.Which alkali metal is the most dangerous?
Cesium and francium are considered the most dangerous due to their extreme reactivity and, in francium’s case, radioactivity.
5.What is the lightest alkali metal?
Lithium is the lightest alkali metal, and it’s also the least dense solid element.
Please don’t forget to leave a review.










